- Home
- About Us
- Services
Bioavailability /
Bioequivalence StudiesMore than 300 completed studies for Indonesian & international sponsors
Clinical Trial
ServicesIn-house trials as well as collaboration with reputable investigators and hospitals
Contract Analysis
ServicesWe offer various analytical testings including chemical, microbiology, physical, and other test
Regulatory Consultation
ServicesExpert guidance for regulatory strategy, submission, and compliance.
- Experience
- Gallery
- Contact Us
- News
- Home
- About Us
- Services
Bioavailability /
Bioequivalence StudiesMore than 300 completed studies for Indonesian & international sponsors
Clinical Trial
ServicesIn-house trials as well as collaboration with reputable investigators and hospitals
Contract Analysis
ServicesWe offer various analytical testings including chemical, microbiology, physical, and other test

Regulatory Consultation
ServicesExpert guidance for regulatory strategy, submission, and compliance.
- Experience
- Gallery
- Contact Us
- News
Publication
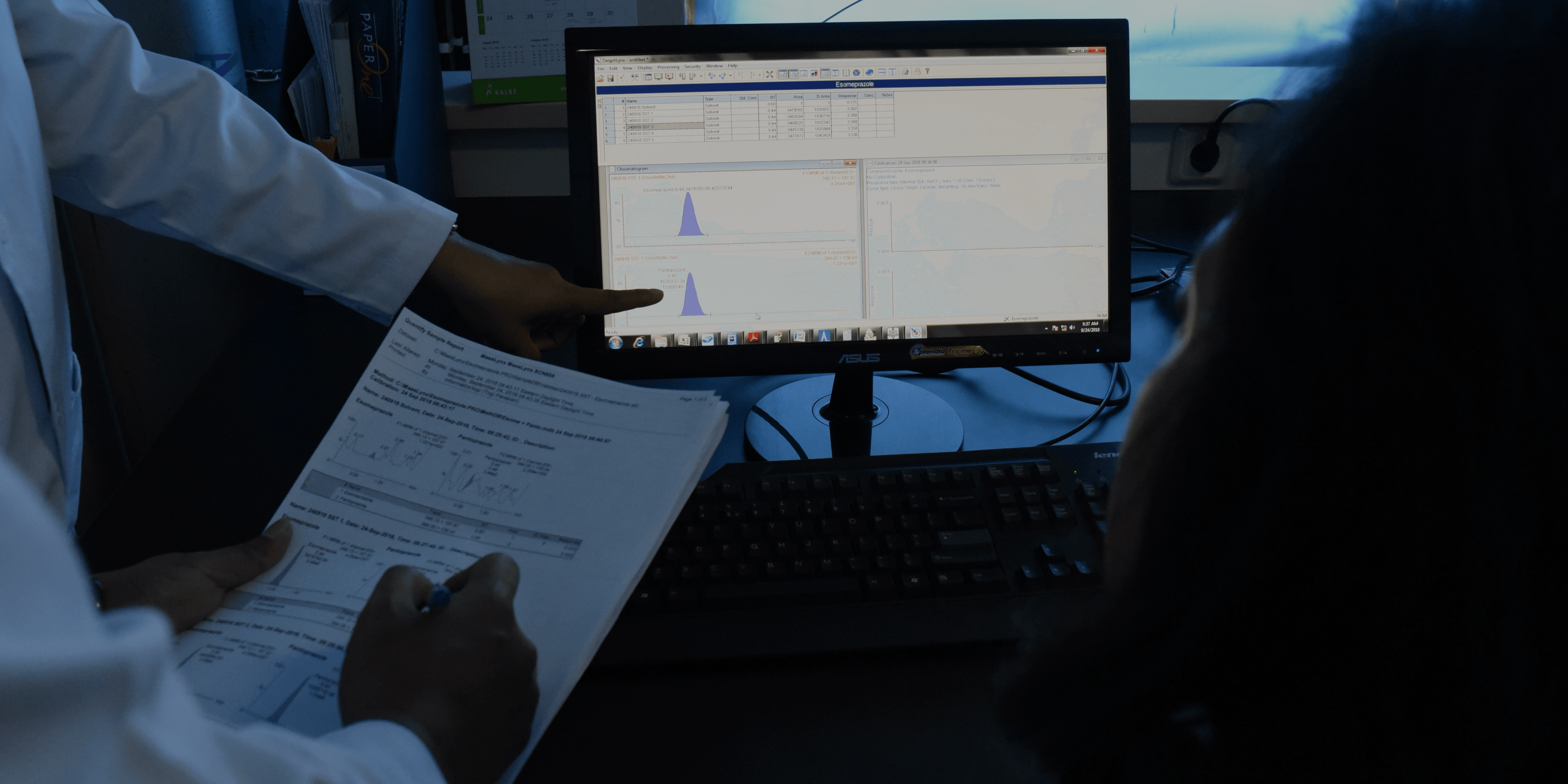
Journal
Bioequivalence Study of Cyproterone Acetate and Ethinyl Estradiol in Healthy Female Volunteers
Published Date: 31 January 2022
The study was conducted to investigate whether 2 mg cyproterone acetate (CPA) and 0.035 mg ethinyl estradiol (EE) film-coated tablet (Elzsa®) manufactured by PT. Sydna Farma was bioequivalent to its reference product, Diane®-35 sugar-coated tablet manufactured by Bayer Weimar GmbH, Germany, imported by PT. Bayer Indonesia, Depok, Indonesia.
Galactooligosaccharide (GOS) Fortified Formula Feeding in Premature Infants
Published Date: 30 November 2021
Nutritional problems are one of the serious problems in low birth weight or preterm infants. This causes medical and nutrition management of premature infants to be more individual. This study showed GOS fortified formula in premature infant formula did not have a detrimental effect on premature infants and did not cause intolerance.

